TCI Organic-Inorganic Perovskite Precursors
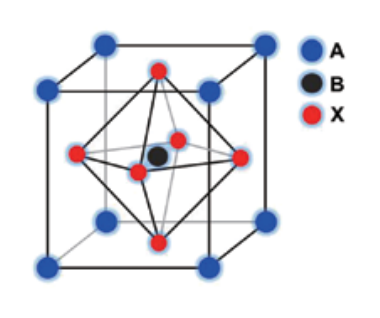
"Perovskite" originates from the mineral name of calcium titanate (CaTiO3) and the compounds with formula of ABX3 generally belong to a perovskite-type compound, where the A is a divalent and B is a tetravalent metal ion. A perovskite with cubic or orthorhombic phases shows ferroelectricity, for instance, barium titanate (BaTiO3) is a ferroelectric or piezoelectric material. High temperature superconductive oxides with a unit of copper oxide are obtained from all perovskite compounds. These perovskite compounds consist of metal ions and oxygen atoms, and are manufactured by a physical procedure (eg. sintering method) Modification of the metal ion and a changing ratio of the metal ion components can drastically control physical properties of the perovskite. In addition to the oxide perovskites, halide-based perovskites are also well known.
On the other hand, one can replace the cationic component with an organic ammonium at the A site. In this case, a chemical method can provide a perovskite compound. This perovskite compound is called an "organic-inorganic perovskite compound", because it contains an organic component. A metal ion component usually involves tin or lead. This perovskite compound has the general formula [(RNH3)mMXn], in which modifications of metal (M), halide (X) and organic groups (R) precisely control physical properties. Among them, the tin perovskite is relatively better for electrical conduction, and the lead one is better for optical properties. A chemical modification of the halide controls band gap. Selection of organic onium halide, metal halide and their mixing ratio changes the component ratio of the halide. The organic groups are selected from methyl, long alkyls, phenyl, benzyl, phenethyl and so on. Diversity of these organic groups allows controlling the structure of a perovskite compound. For instance, a perovskite compound with R = methyl provides [(MeNH3)MX3] having a three-dimensional cubic perovskite structure. A perovskite compound with R = CnH2n+1 (n ≥ 2) provides a twodimensional perovskite layer and the length of alkyl group can control the inter-layer distance.
TCI Organic Transistor (OFET) Materials
Organic field-effect transistors (OFETs) are promising components for the next-generation electronic devices. In 1984, OFET research began with the first report of mobility in a merocyanine dye-based field-effect device by Kudo et al. Since high hole mobility (1.5 cm2 /Vs), which comparable to that of amorphous silicon, was achieved using a pentacene-based OFET device in 1997, the possibility of a practical application of OFET’s became realistic and the research field became quite popular worldwide. While silicon has performed well in devices, the inorganic nature of them prohibits flexible structures. As a result, OFETs have attracted much attention for their potential to be flexible, thin and light-weight, which could be applicable for foldable electronic circuits and implantable biometric sensors.
A noted potential application with OFET’s involves their “printability”. Printed electronics are an innovative technology for mass and low cost device productions, which could allow for the production of high density and large circuits on flexible substrates such as paper and plastic films. A fusion of “Printed electronics” and “Organic transistors” could offer especially promising technology by allowing for low cost and large scale manufacture of various functional devices.
One of the functional parameters evaluated in organic semiconductor materials is mobility (μ), which indicates how fast the holes (p-type) or electrons (n-type) within the semiconducting layer move. A material that possesses high carrier mobility is required for producing high-speed circuits. OFET devices are a largely simple construction containing an organic semiconductor layer, an insulating layer, and source-drain-gate electrodes. These components allow for the evaluation of fundamental transistor parameters including mobility, operation voltage, and driving stability.
TCI Organic Light-Emitting Diode (OLED) Materials

Organic light-emitting diode (OLED) devices have received much attention, because they are expected to be a next generation display and light source, thanks to lightweight and flexible organic materials. The OLED was focused on practical use, after Tang et al first observed the OLED device by use of a two layered organic thin film. Adachi et al further reported a three layered device, in which a host layer is sandwiched by hole transport and electron transport layers. In addition, they reported a two layered system, in which one layer has roles of host and electron transport properties. A five layered system including electron injection and hole injection layers has been also studied in order to improve the efficiency of carrier injection. One can control RGB colors of emission by selection of a dopant into a host layer. A suitable combination of the dopant can give a white colored device. An application using the white organic light-emitting device (WOLED) is an OLED light panel.
An amorphous material is useful for an OLED device, because it is transparent, homogeneous, isotropic and easily processible. A practical OLED device further requires excellent heat-resistance and durability. Many hole transport materials based on triphenylamine derivatives (TPD) are widely usable, because they are heat-resistant and amorphous. In addition to the TPDs, oxadiazole derivatives (PBD) having an electron transport property,9) Alq3 as a host material, and blue emissive distylyl derivatives are fundamental materials for amorphous OLED devices.
TCI Mitsunobu Reaction

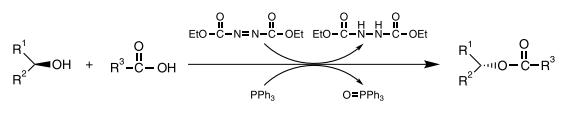
In 1967 Mitsunobu reported the reaction of alcohols and carboxylic acids in the presence of diethyl azodicarboxylate (DEAD) and triphenylphosphine (TPP) to give the corresponding esters in high yield.1) This reaction involves the activation of an alcoholic hydroxyl group and the subsequent carbon-oxygen bond cleavage caused by an attacking carboxylate anion, to give an ester with complete Waldeninversion of the alcohol stereocenter. Furthermore, carboxylic acids are not the only nucleophiles which can be used in this reaction. Imides and thiols can also be used as the nucleophilic component. This reaction constitutes one of the most important organic reactions, and it is therefore called the "Mitsunobu reaction" after its developer.
The Mitsunobu reaction has found widespread use in many fields because of its high reliability and extensive versatility. For example, searching SciFinder® for the keyword "Mitsunobu," from 1967 to today, one encounters about 4,500 related reports, indicating the high utility of this reaction. However, the generation of phosphine oxide and hydrazinedicarboxylate as by-products often makes the isolation of pure product difficult. Furthermore, the pKa of the usable acidic component must be below 13, but preferably below 11. Since the Mitsunobu reaction has its versatility, efforts have been made toward widening the utilization scope.
TCI Ionic Liquids
As seen above, ionic liquids are salts, consisting of cations such as imidazolium, pyridinium, quaternary ammonium and quaternary phosphonium, and anions such as halogen, triflate, tetrafluoroborate and hexafluorophosphate, which exist in the liquid state at relatively low temperatures. Their characteristic features include almost no vapor pressure, non-flammability, non-combustibility, high thermal stability, relatively low viscosity, wide temperature ranges for being liquids, and high ionic conductivity. When an ionic liquid is used as a reaction solvent, the solute is solvated by ions only, where the reaction proceeds under quite different conditions as compared to using water or ordinary organic solvents. Hence, they are expected to exhibit unconventional reactivity, and their applications in a variety of organic reactions are being explored.
Ionic liquids containing chloroaluminate as the anion have been investigated for many years. These ionic liquids are not only used as reaction solvents, but also exhibit Lewis acid or Lewis base properties, when the ratio of cations and anions is changed. However, they can only be used under an inert atmosphere or vacuum, due to their high moisture sensitivity. On the other hand, it has been found that ionic liquids containing anions such as hexafluorophosphate form stable salts in air, which lead to the synthesis of numerous stable ionic liquids today. Furthermore, some ionic liquids have very low solubility in water and polar organic solvents. Utilization of this property enables recovery and reuse of ionic liquids, after extracting the product with an organic solvent. That can help to reduce the waste of traditional solvents which are rarely reused. Moreover, ionic liquids have attracted much attention as safe solvents, due to their low volatility.
TCI Building Blocks for Organic Semiconductor

Light and flexible organic semiconductor materials are promising for foldable electronic circuits1) and implantable biometric sensors,2) although such electronic devices are hardly obtained from silicon-based semiconductors. We have developed a printed electronics system giving large scale and highly precise devices on flexible substrates (eg. paper and film) by a printing method thanks to the solubility of organic materials. The printing method is one efficient technology for mass production and low cost production of semiconducting devices.3) Organic photovoltaics (OPV) is a photoelectric conversion device using organic semiconductors. Organic light-emitting diode (OLED) devices have received much attention, because they are expected to be a next generation display and light source, thanks to lightweight and flexible organic semiconductor materials. Organic semiconductor materials are mainly classified into small molecular and polymer types, and an oligomer type is a middle class between them.
TCI Fluorinating Agents, Building Blocks, Fluorinated Bioactive Compounds

Since there are few naturally occurring fluorine containing compounds, it is necessary to fluorinate organic compounds at a certain stage of the syntheses. Though fluorine gas and hydrogen fluoride are used as fluorine sources, they are very toxic and corrosive and require special equipment and techniques for handling them. Therefore, alternate fluorinating agents have been developed, which are used in laboratories to easily introduce a fluorine atom at selected positions of the compounds. The fluorinating agents are roughly classified into two categories: nucleophilic and electrophilic. Nucleophilic fluorinating agents are those where the fluoride anion serves as a reaction active species. Electrophilic fluorinating agents are those where the electron-deficient fluorine atom serves as a reaction active species. In addition, a fluorinated building block is used as a fluorine source, which has both a fluorine atom and a replaceable functional group in a molecule.
TCI Cosmetic Materials

TCI Chemical Compounds Purified by Sublimation
Reagent purity is paramount in countless applications, and is of particular importance when involving electronic materials including polyaromatic hydrocarbons. Pentacene (P0030, P2524) and N,N’-diphenyl-N,N’-di(m-tolyl)-benzidine (D3236) in particular have garnered increased interest for their applications in organic semiconducting materials. The purification of organic compounds is almost entirely conducted via column chromatography or recrystallization. These mainstay procedures are highly effective, but are limited to compounds soluble in organic solvents.
Many electronic materials including PAHs due to their extensive π conjugation and aromaticity are poorly or completely insoluble in organic solvents. Fortunately, this very π conjugation lends to both high volatility with a relatively high sublimation temperature lending to sublimation purification being a highly effective purification avenue. To address research and industry demands, TCI has introduced several electronic materials purified by sublimation which can be readily and reliably used in a variety of research applications.
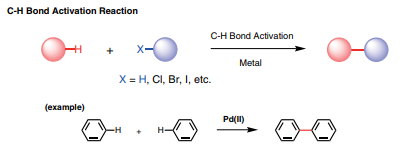
TCI C-H Bond Activation Reaction
Other Products Available from Greyhound

If you know our company you will know that for over 38 years Greyhound has been supplying high quality Chromatography consumables to laboratories around the world. We hold ISO 9001:2008 Certificate No: RS 30332 for the supply and stockholding of analytical reference standards, chemicals, chromatography columns and related accessories. Our Certified Reference Standards are supplied by Guide 34 accredited manufacturers.
If you don’t know us, please visit our website www.greyhoundchrom.com we have access to over 1 million products – including the largest catalogue of Certified Reference and Analytical standards in the world.
Greyhound’s extensive range covers all areas of Agriculture, Chemical, Environmental, Food, Forensics, Petrochemical, Chemical, Life Sciences and Pharmaceutical analysis, holding stock of many popular products for prompt delivery via our extensive logistics network.
Greyhound prides itself on personal service which provides prompt, efficient, cost-effective, safe delivery of all products. With state-of-the art facilities and highly trained staff, Greyhound provides technical advice and distribution of Chromatography consumables across all disciplines. Our service is designed to provide a wide range of products, to help our clients to achieve excellent, cost-effective results. Greyhound manufactures its own range of Capillary Columns, Syringe Filters, SPE Columns and HPLC Columns, the 'Q' Range, as well as representing the industry’s best known manufacturers.
By working with the industry’s best known manufacturers we are able to tailor products to customer requirements and supply products on behalf of 3M, Biosolve; Chiron; Chromacol; Chem Service (Environmental and Pesticides); EP Scientific; Extrasynthese, Hach Lange, Hamilton; High Purity Standards; Jour Research; Larodan Fine Chemicals; Macherey-Nagel; Millipore, National Scientific; NIST, PAH Reference Standards; Pfaltz and Bauer; Regis Technologies; Rheodyne; RT Corporation; Samco Scientific; SGE; SGT Filters; Sigma Aldrich, Fluka and Supelco, Sillicycle, Speciality Gases, Swagelok, TCI, Thermo, Upchurch; Valco, Vici , Wellington Laboratories and Wheaton. We offer a full range of chromatography and laboratory consumables, if you visit our website and do not see what you require please contact us and we will do our best to source your requirements for you.
Our services include; Chemical Standards; Chiral Chromatography; Derivatives Analysis; Solvents; Dioxins/Furans/PCBs/PBDEs; Environmental Analysis; Gas Filters; GC; GC-MS; LC-MS; HPLC; ICP, ICP-MS, AA; Ion Chromatography; Lamps; Prep Silica; Reagents; SPE Solid Phase Extraction; SPME Solid Phase Micro-Extraction; Syringes; TLC Thin Layer Chromatography; Valves; Vials/Caps/Septa. Our sales team is available to discuss your requirements in detail, from the application of products, to sourcing and prompt delivery. We are able to source hard to find chemicals which are no longer commercially available and are able to supply over 13,000 laboratory chemicals in small, convenient units.
CONTACT US
Tel: +44 (0) 151 649 4000
Email: marketing@greyhoundchrom.com
FOLLOW US
YOU MAY ALSO BE INTERESTED IN OUR NEWSLETTER

 Materials SNIP.PNG)
 Materials SNIP.PNG)