Water determination in aldehydes and ketones by Honeywell
HYDRANAL™ Laboratory Report L 676

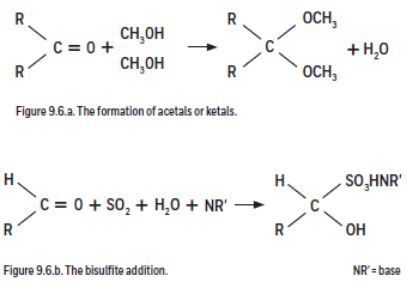
Both aldehydes and ketones pose problems with Karl Fischer titration because they form acetals and ketals respectively with conventional KF reagents (Figure 9.6.a). The reaction forms water, which is also titrated, resulting in vanishing end points and erroneously high water content. With aldehydes a second side reaction, the bisulfite addition, can also occur (Figure 9.6.b). This reaction consumes water and leads to an erroneously low water content.
The amount of water to be titrated should be low enough so that titrations are not inordinately long. We found sample sizes that contain a total of 10–25 mg H2O are ideal. This amount of water consumes 2–5 mL reagent. We have investigated the moisture determination of a number of aldehydes and ketones during the development of the Hydranal-K reagents. These compounds are
listed in Table 9.6. The table shows the name of the chemical and the water content. The water content given is for reference only and is not to be taken as a limit. Column 3 lists the size of the sample that can be titrated in a 25 mL volume of Hydranal-Working Medium K or Hydranal-Medium K. The entry ‘10 mL’ or ‘10 g’ represents the largest sample size analyzed. Smaller sample sizes
are indicated in column 4 with a designation for the reason of the limited sample size:
B = bisulfite addition
I = indication interferences
L = limited solubility
A = buffering of acid
The data in Table 9.6 shows that the determination of water in most ketones is straightforward. 10 mL samples of aliphatic ketones can be titrated without any interference, even with acetone and cyclohexanone, which are particularly reactive. However, trifluoroacetone gives a noticeable bisulfite addition reaction, so a ‘flying start’ titration and a verification of end point are required for its reliable determination. Aromatic ketones and long-chain aliphatic ketones are less reactive and can also be titrated with Hydranal-Composite 5 and Hydranal-Medium K or Hydranal-Working Medium K or Hydranal-KetoSolver or Hydranal-CompoSolver E (the use of Hydranal-Composite 5 K is not necessary). Most heterocyclic ketones perform similarly to aromatic ketones. With acetyl pyridine the bisulfite addition is apparently activated by the pyridyl group, and therefore interferes with the water determination.
Diketones usually behave like normal ketones. Exceptions are diacetyl ketone and 1,2-cyclohexanedione to a certain extent. The adjacent keto groups, particularly in diacetyl ketone, are very reactive and only small amounts of sample can be analyzed. This is not the case with benzyl ketone presumably due to the aromatic substituents present in this compound. Keto-carboxylic acids shift the pH of the working medium and delay the course of the titration. Buffering the working medium slightly accelerates the titration and restores the pH.

CONTACT US
Tel: +44 (0) 151 649 4000
Email: marketing@greyhoundchrom.com
FOLLOW US
YOU MAY ALSO BE INTERESTED IN OUR NEWSLETTER















